Element vs Compound: What's the Scientific Difference?

Written by
Ernest Bio Bogore

Reviewed by
Ibrahim Litinine
Understanding the fundamental building blocks of matter isn't just academic exercise—it's the foundation for comprehending everything from pharmaceutical development to materials engineering. An element represents the purest form of matter, consisting of atoms with identical atomic numbers, while a compound emerges when two or more different elements bond chemically in fixed proportions. This distinction drives every chemical reaction, manufacturing process, and biological function around us.
Consider why this matters beyond textbooks. Every medication you take, every material in your smartphone, every breath you draw involves the interplay between elements and compounds. Oxygen exists as an element in your lungs, but becomes part of water compounds in your bloodstream. Silicon stands alone as an element in computer chips, yet transforms into silicon dioxide compounds in glass. These aren't abstract concepts—they're the operational principles governing our technological civilization.
What's the main difference between elements and compounds?
The fundamental distinction lies in atomic composition and chemical behavior. Elements cannot be broken down through chemical means because they consist entirely of atoms sharing the same atomic number—the same number of protons in their nuclei. Compounds, conversely, result from chemical bonds between different elements, creating substances with properties entirely different from their constituent parts.
This difference manifests in three critical ways that impact real-world applications. First, elements maintain consistent properties regardless of source or quantity. Pure carbon exhibits identical characteristics whether extracted from diamond, graphite, or coal. Second, compounds display emergent properties—characteristics absent in their component elements. Sodium explodes in water, chlorine gas proves lethal, yet sodium chloride (table salt) safely seasons food. Third, separation methods differ fundamentally. Elements require nuclear processes for alteration, while compounds break apart through chemical reactions.
The implications extend beyond laboratory curiosity. Pharmaceutical companies leverage this principle when designing drug compounds. They combine elements like carbon, hydrogen, oxygen, and nitrogen in specific arrangements to create molecules that interact with biological systems in predetermined ways. A slight change in elemental composition—adding or removing a single atom—can transform a life-saving medication into an ineffective or dangerous substance.
Manufacturing industries depend on this distinction for quality control. Steel production requires precise control over elemental composition, determining whether the final product becomes surgical instruments or construction beams. The difference between 0.3% and 0.8% carbon content in iron compounds determines whether you get mild steel for car bodies or high-carbon steel for cutting tools.
How and when to use elements and compounds with examples?
Elements find application when purity and specific atomic properties prove essential. Industrial processes requiring precise electrical conductivity rely on elemental copper rather than copper compounds. The semiconductor industry demands ultra-pure silicon elements because even trace compound formation would compromise electronic properties. Medical imaging uses elemental contrast agents like barium or iodine, where the specific atomic structure creates the desired X-ray opacity.
Timing becomes crucial in elemental applications. Welding operations use elemental argon gas during the process because its inert nature prevents unwanted chemical reactions, but this protection only works when compounds haven't formed. Food packaging employs elemental nitrogen to prevent oxidation, but once oxygen compounds form, the protective effect disappears.
Compounds serve different strategic purposes, particularly when enhanced or novel properties are required. Water represents the most essential compound—two hydrogen atoms bonded to oxygen create properties neither element possesses individually. Hydrogen burns explosively, oxygen supports combustion, yet their compound extinguishes fires. This emergent behavior makes compounds invaluable for specialized applications.
Pharmaceutical development exemplifies compound timing considerations. Aspirin (acetylsalicylic acid) requires specific elemental bonding to achieve its anti-inflammatory effects. The compound must remain stable during storage but break down predictably in the human body. If elemental separation occurs prematurely, therapeutic benefits disappear.
Construction materials demonstrate compound utility in structural applications. Concrete combines calcium compounds with silicate compounds to create materials stronger than either component alone. The chemical bonding between different elemental groups produces a composite that resists compression forces while maintaining workability during installation.
Real-life scenarios where elements and compounds are used
Scenario 1: Emergency Medical Treatment When a patient suffers carbon monoxide poisoning, emergency responders administer pure elemental oxygen through high-flow masks. The treatment exploits elemental oxygen's higher binding affinity compared to carbon monoxide compounds. Healthcare workers cannot substitute oxygen compounds like hydrogen peroxide because the bound oxygen lacks the immediate availability necessary for reversing cellular hypoxia. The elemental form provides direct therapeutic intervention where compound alternatives would prove ineffective or dangerous.
Scenario 2: Semiconductor Manufacturing Quality Control Silicon chip fabrication requires elemental silicon with purity levels exceeding 99.9999%. Manufacturing engineers monitor for compound formation throughout the process because even microscopic silicon dioxide formation creates electrical defects. When silicon reacts with trace oxygen to form compounds, the resulting material changes from conductor to insulator, rendering entire chip batches unusable. Quality control systems specifically test for elemental purity versus compound contamination.
Scenario 3: Corporate Food Safety Compliance Food processing companies use sodium chloride compounds for preservation, but cannot substitute elemental sodium or chlorine. The compound provides antimicrobial properties through osmotic pressure, while maintaining consumer safety. Elemental sodium would react violently with moisture, creating explosions. Elemental chlorine would poison consumers. Only the specific compound delivers the required preservation effect while meeting safety regulations and taste requirements.
Scenario 4: Pharmaceutical Patent Strategy Biotech companies develop proprietary drug compounds by modifying existing elemental arrangements. A depression medication might combine carbon, hydrogen, nitrogen, and oxygen in a novel configuration that produces superior therapeutic effects compared to existing compounds. The specific elemental bonding pattern becomes intellectual property, protecting the investment in research and development. Generic manufacturers cannot replicate the compound without violating patents, even though they have access to the same elements.
Common mistakes to avoid when using elements and compounds
Mistake 1: Assuming Similar Names Indicate Similar Properties Students frequently confuse elemental hydrogen with hydrogen compounds, expecting similar behavior. Elemental hydrogen gas proves highly flammable and requires specialized storage, while hydrogen compounds like water or hydrogen peroxide exhibit completely different safety profiles and applications. This confusion leads to improper handling procedures and safety violations in laboratory settings. The element requires explosion-proof equipment and inert atmospheres, while many hydrogen compounds can be handled with standard chemical safety protocols.
Mistake 2: Interchanging Elements and Compounds in Industrial Applications Manufacturing personnel sometimes substitute compounds for elements without understanding performance implications. Using iron oxide (rust) instead of elemental iron in metallurgical processes produces inferior alloys with compromised strength characteristics. The compound requires additional reduction steps to extract the elemental iron, adding cost and complexity. Similarly, attempting to use carbon compounds instead of elemental carbon for filtration applications reduces effectiveness because the bonded carbon lacks the surface area and adsorption properties of the pure element.
The critical distinction involves understanding that compounds require breaking existing bonds before forming new ones, while elements can form bonds directly. This difference affects reaction kinetics, energy requirements, and final product properties across industrial applications.
4 alternative terms for elements and compounds
Pure Substances vs Chemical Combinations Pure substances emphasize the homogeneous nature of elements—every atom identical in nuclear composition. This terminology proves particularly useful in analytical chemistry where contamination assessment requires distinguishing between pure elemental content and chemical combinations. Laboratory technicians use "pure substance" when referring to elements that haven't undergone chemical bonding, while "chemical combination" indicates compound formation has occurred.
Atomic Species vs Molecular Entities Atomic species highlights the fundamental particle nature of elements, focusing on the specific nuclear configuration that defines each element's identity. Molecular entities encompasses both simple compounds (like water) and complex compounds (like proteins), emphasizing the structural arrangement of different atomic species. Research publications often use this terminology when discussing reaction mechanisms and structural analysis.
Elementary Matter vs Composite Materials Elementary matter stresses the indivisible chemical nature of elements—they cannot be broken down further through chemical processes. Composite materials emphasizes the multi-component nature of compounds, highlighting how different elements contribute distinct properties to the final substance. Engineering specifications frequently employ this terminology when describing material requirements and performance characteristics.
Monoatomic Substances vs Polyatomic Substances This terminology proves most precise for advanced applications. Monoatomic substances consist of single-element atoms, whether existing individually (like noble gases) or in same-element groups (like oxygen gas, O₂). Polyatomic substances contain multiple different elements bonded together, creating new chemical identities. Academic research and industrial patents often use this terminology for technical precision.
Why This Distinction Drives Scientific Innovation
The element-compound relationship fuels technological advancement across industries. Pharmaceutical companies invest billions developing new compounds because slight modifications in elemental arrangement can produce breakthrough treatments. The COVID-19 vaccines emerged from understanding how specific compound modifications could trigger desired immune responses while avoiding harmful side effects.
Materials science advances similarly depend on this fundamental distinction. Graphene represents elemental carbon arranged in specific patterns, creating materials stronger than steel yet flexible as rubber. The same carbon element, arranged differently in diamond compounds, produces the hardest known natural material. These aren't coincidental variations—they demonstrate how elemental properties combine with structural arrangements to create revolutionary materials.
Advanced Applications in Modern Technology
Quantum computing represents the cutting edge of element-compound manipulation. Quantum processors require ultra-pure elemental materials like silicon for substrate foundations, combined with precisely engineered compounds for quantum dot formation. The technology succeeds only when engineers maintain absolute control over which atoms exist as elements versus compounds, because quantum effects disappear when unwanted chemical bonds form.
Battery technology similarly exploits element-compound relationships. Lithium-ion batteries use elemental lithium for ion transport while relying on lithium compounds for energy storage. The charging process converts compounds back to elements and vice versa, storing and releasing energy through controlled chemical bonding. Next-generation solid-state batteries promise superior performance by optimizing these element-compound transitions.
Environmental and Safety Implications
Understanding elements versus compounds proves crucial for environmental protection and workplace safety. Heavy metal contamination involves elemental forms like mercury or lead, requiring different remediation strategies than compound contamination. Elemental mercury evaporates at room temperature, creating vapor hazards, while mercury compounds may be solid but pose different toxicity risks through ingestion or skin contact.
Industrial safety protocols must account for these differences. Storage requirements, handling procedures, and disposal methods vary dramatically between elements and compounds. Elemental sodium requires oil storage to prevent water contact, while sodium compounds may be safely stored in air. These distinctions prevent accidents and ensure regulatory compliance across industries.
The mastery of element-compound relationships ultimately determines success in fields ranging from medicine to engineering to environmental science. As technology advances, this fundamental distinction becomes increasingly important for innovation, safety, and sustainable development.
Learn Any Language with Kylian AI
Private language lessons are expensive. Paying between 15 and 50 euros per lesson isn’t realistic for most people—especially when dozens of sessions are needed to see real progress.
Many learners give up on language learning due to these high costs, missing out on valuable professional and personal opportunities.
That’s why we created Kylian: to make language learning accessible to everyone and help people master a foreign language without breaking the bank.
To get started, just tell Kylian which language you want to learn and what your native language is
Tired of teachers who don’t understand your specific struggles as a French speaker? Kylian’s advantage lies in its ability to teach any language using your native tongue as the foundation.
Unlike generic apps that offer the same content to everyone, Kylian explains concepts in your native language (French) and switches to the target language when necessary—perfectly adapting to your level and needs.
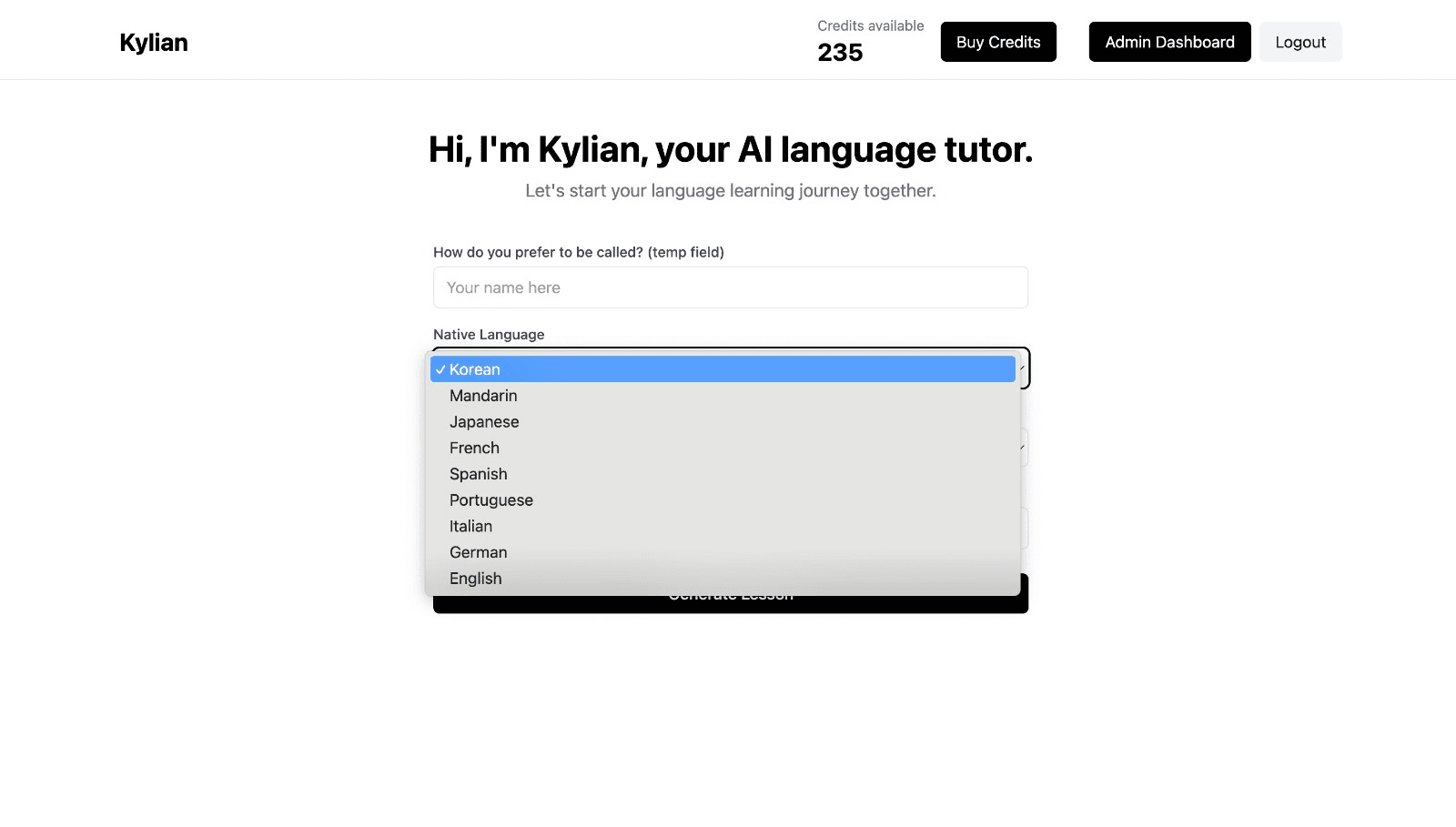
This personalization removes the frustration and confusion that are so common in traditional language learning.
Choose a specific topic you want to learn
Frustrated by language lessons that never cover exactly what you need? Kylian can teach you any aspect of a language—from pronunciation to advanced grammar—by focusing on your specific goals.
Avoid vague requests like “How can I improve my accent?” and be precise: “How do I pronounce the R like a native English speaker?” or “How do I conjugate the verb ‘to be’ in the present tense?”
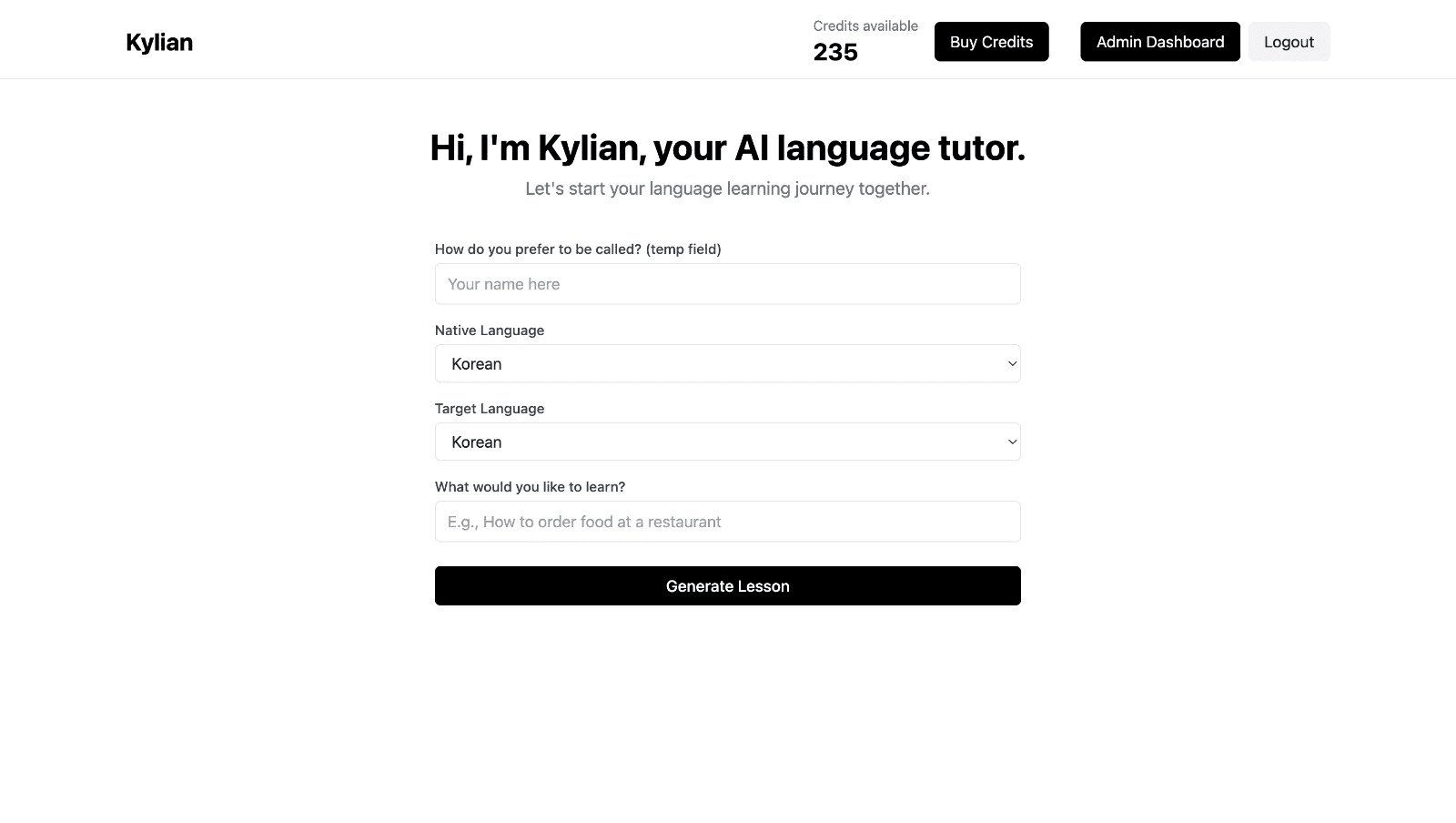
With Kylian, you’ll never again pay for irrelevant content or feel embarrassed asking “too basic” questions to a teacher. Your learning plan is entirely personalized.
Once you’ve chosen your topic, just hit the “Generate a Lesson” button, and within seconds, you’ll get a lesson designed exclusively for you.
Join the room to begin your lesson
The session feels like a one-on-one language class with a human tutor—but without the high price or time constraints.
In a 25-minute lesson, Kylian teaches exactly what you need to know about your chosen topic: the nuances that textbooks never explain, key cultural differences between French and your target language, grammar rules, and much more.
Ever felt frustrated trying to keep up with a native-speaking teacher, or embarrassed to ask for something to be repeated? With Kylian, that problem disappears. It switches intelligently between French and the target language depending on your level, helping you understand every concept at your own pace.
During the lesson, Kylian uses role-plays, real-life examples, and adapts to your learning style. Didn’t understand something? No problem—you can pause Kylian anytime to ask for clarification, without fear of being judged.
Ask all the questions you want, repeat sections if needed, and customize your learning experience in ways traditional teachers and generic apps simply can’t match.
With 24/7 access at a fraction of the cost of private lessons, Kylian removes all the barriers that have kept you from mastering the language you’ve always wanted to learn.
Similar Content You Might Want To Read

Tart or Sour? Understand the Flavor Distinction in English
The human palate recognizes five primary taste sensations: sweet, salty, bitter, umami, and sour. Yet within these classifications lie subtle variations that languages around the world attempt to capture with remarkable precision. Among the most frequently confused flavor descriptors in English are "tart" and "sour" - two terms that, while related, convey distinct sensory experiences. This confusion extends beyond mere semantics; it affects how we communicate about food, how culinary professionals develop recipes, and ultimately shapes our expectations and experiences with flavors. The distinction between tart and sour represents a fascinating intersection of sensory perception, language, and cultural context that merits deeper examination.

Communication Acronyms You Need to Know
Communication has evolved dramatically with technology, creating a lexicon of abbreviations that shape how we interact professionally and personally. These acronyms aren't just shortcuts—they're efficiency tools that, when used correctly, can enhance clarity and speed in our exchanges. Mastering communication acronyms matters because miscommunication costs organizations an average of $37 billion annually according to the Society for Human Resource Management. Understanding these abbreviations eliminates confusion, accelerates decision-making, and demonstrates professional competency in modern workplace environments.
![Service Abbreviations You Need to Know [Complete Guide]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F147z5m2d%2Fproduction%2F3652a055cfe6cb268c3f3ae905793cef9bc4a0aa-2240x1260.png%3Frect%3D175%2C0%2C1890%2C1260%26w%3D600%26h%3D400&w=3840&q=75)
Service Abbreviations You Need to Know [Complete Guide]
Communication efficiency drives business success. When professionals navigate service-related conversations, documents, and contracts, understanding abbreviations becomes critical for avoiding misunderstandings and maintaining credibility. Service abbreviations appear everywhere: invoices, contracts, technical documentation, customer support tickets, and professional correspondence. Misinterpreting "SLA" as "Service Level Agreement" versus "Special Leave Allowance" can derail entire project timelines. The stakes are higher than many realize. In this article, we'll decode the most essential service abbreviations across industries, explain their contextual usage, and provide practical guidance for professional application.

Emergency Abbreviations: Critical Acronyms to Know
Emergency situations demand precision, speed, and clarity. When seconds matter, professionals across healthcare, emergency services, and crisis management rely on standardized abbreviations to communicate critical information efficiently. These acronyms serve as linguistic shortcuts that can mean the difference between life and death. Understanding emergency abbreviations becomes essential whether you work in healthcare, emergency services, or simply want to comprehend the terminology used during critical situations. In this article, we'll explore the most vital emergency abbreviations, their proper usage, and the contexts where they prove indispensable.

New England Abbreviation: Different Codes & Acronyms
Understanding regional abbreviations becomes crucial when navigating business communications, official documents, or casual correspondence across America's northeastern corridor. New England's unique position as both a historical region and modern economic hub generates countless abbreviations that professionals and residents encounter daily. Whether you're relocating to Massachusetts, conducting business in Connecticut, or simply trying to decode a Vermont address, mastering these abbreviations eliminates confusion and demonstrates regional awareness. The complexity increases when you consider that New England encompasses six distinct states, each with its own governmental, educational, and cultural institutions that generate their own abbreviated forms.
![50+ Key Business Abbreviations Decoded [English]](/_next/image?url=https%3A%2F%2Fcdn.sanity.io%2Fimages%2F147z5m2d%2Fproduction%2F86cecc24a7758c8a61c341d0bd0aa33e20ea130b-2240x1260.png%3Frect%3D175%2C0%2C1890%2C1260%26w%3D600%26h%3D400&w=3840&q=75)
50+ Key Business Abbreviations Decoded [English]
Navigating the corporate landscape requires fluency in a specialized language—one filled with shorthand expressions that convey complex ideas efficiently. Business abbreviations serve as the cornerstone of professional communication, enabling teams to exchange information rapidly without sacrificing clarity. This guide unpacks over 50 critical acronyms you'll need to master business English and communicate with confidence in any professional setting.